QUALITY OF CARE, MANAGEMENT OF SURVEILLANCE AND RISKS
Lacassagne has been committed since 1999 to a rigorous quality and risk management policy to achieve a high level of care. Quality has been a constant concern for all Center staff for many years. To this end, it follows an ambitious quality and risk management policy, implemented with professionals from each of the institution’s units, guided and supported by the Quality / Risk Department, the Vigilance and Risks Committee and the Medical Establishment Commission.
This commitment is measured by certification and accreditation visits. The certification of the High Authority of Health is a mandatory procedure for all public and private hospitals in France, whereby the quality of services offered to patients is evaluated. The Center was certified by the High Authority for Health in 2002, 2006 and 2012. In 2016, for the 4th time in a row, it obtained the highest level of certification.
CAL was also accredited by COFRAC for its laboratory activities in 2015, certified in 2015 for its tumor biobank. Currently, the Department of Clinical Research and Innovation is also preparing its certification visit.
The Quality Department works in close collaboration with numerous committees such as the Nosocomial Infection Control Committee, the Anti-Pain Committee and the User Commission as well as with the Management of Communication Center with which it develops clear, understandable information for patients. The patient information documents are written by the care teams and read by user representatives prior to validation by the Quality Risk Committee.
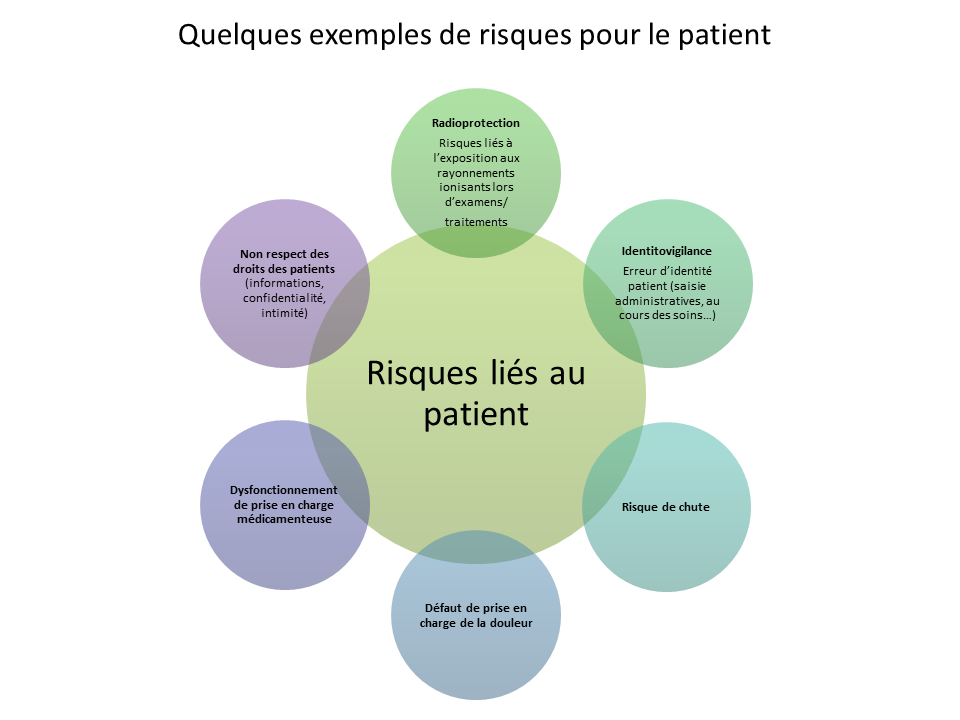
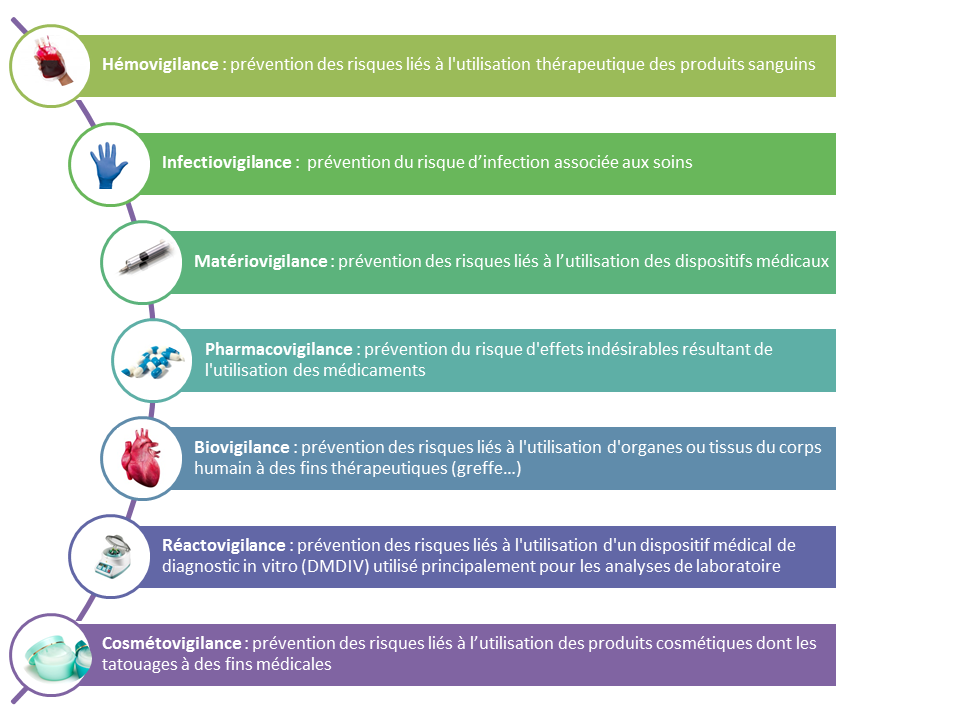
Surveillance and risk management is a cross-cutting approach that aims to identify, evaluate and reduce, whenever possible, the risks incurred by patients, visitors and staff. It improves the safety of patient care in many areas: drugs, anesthesia, blood transfusion, identitovigilance, healthcare equipment … A Coordination Committee for Health Surveillance and Risk Management monitors risks within the establishment and unites with the Quality Department and the President of the GCE, all the Health Watch and Risk Managers.
All professionals, patients, and loved ones must contribute.
Please report any incident related to your care to the professionals who take care of you: this may concern an examination, a treatment, a hotel service dysfunction … Any undesirable event can thus be recorded, reported, analyzed, treated and Appropriate preventive measures will be taken to protect other patients from a similar incident. Improving the safety of care is based on the reporting of incidents and adverse effects related to care. This statement is intended to improve your safety and that of all patients.